Answers
Answer:
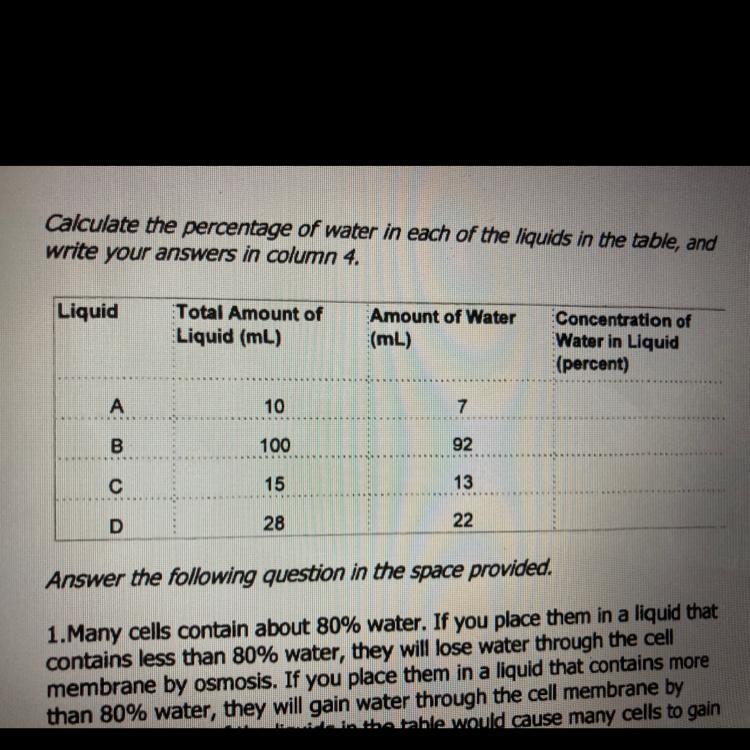
A 70%
B 92%
C 87%
D 79%
Explanation:
This excercise is about dividing water by liquid.
Related Questions
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) In the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions.
Answers
Answer:
Overall enthalpy change for the formation of one mole nitric acid from nitrogen, hydrogen and oxygen, ΔH = -376 KJ
Note: the question is incomplete. The complete question is given below:
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N₂(g) + 3H₂(g) → 2NH₃(g) ΔH = -92. kJ In the second step, ammonia and oxygen react to form nitric acid and water: NH3(g) + 2O2(g) → HNO3(g) + H2O(g) ΔH = -330. kJ Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ.
Explanation:
From Hess's law of constant heat summation, the total enthalpy change for a reaction is the sum of all changes without regard to the number of multiple stages or steps involved in a reaction.
Enthalpy is a state function as it does not depend on the path taken to attain its value. Therefore, the summation of the enthalpy changes involved in the individual steps in the reaction of the formation of nitric acid will be equal to the enthalpy change of the overall reaction step.
For the first reaction step:
N₂(g) + 3H₂(g) → 2NH₃(g) ΔH = -92. kJ
For the second reaction step:
NH₃(g) + 2O₂(g) → HNO3(g) + H2O(g) ΔH = -330. kJ
Overall reaction step:
[tex]\frac{1}{2}[/tex]N₂(g) + [tex]\frac{3}{2}[/tex]H₂(g) + 2O₂(g) → HNO₃ + H₂O ΔH = ?
The overall reaction for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen shows that the first reaction step should be divided by 2
[tex]\frac{1}{2}[/tex]N₂(g) + [tex]\frac{3}{2}[/tex]3H₂(g) → NH₃(g) ΔH = -46. kJ
Overall enthalpy change, ΔH = ΔH₁ + ΔH₂
Overall enthalpy change,ΔH = (-46 KJ) + (-330 KJ)
Overall enthalpy change,ΔH = -376 KJ
You pump 100 gas particles in Basketball A and 100 gas particles in Basketball B. both basketballs are at room temperature ¿Which basketball will be more firm? ¿And Why?
Answers
Answer:
I think the answer is probably b
Which of the following is a characteristic of a scientific theory? A.it explains how nature works. B.it is based on a single experiment. C.it should not be possible to replicate it's results. D.it should not be possible to replicate it's observations.
Answers
Answer:
A. It explains how nature works.
Explanation:
A scientific theory is an explanation, based on a body of evidence, of how something works or will behave.
The other three answers contradict scientific theory. Theories should be based on many experiments, and you should be able to replicate the results and observations.
What is the mole ratio of nitrogen (N2) to ammonia (NH3)?
N2 + 3 H2 → 2 NH3
a.2:3
b3:1
c.3:2
d.1:3
Answers
The only three colors of light the human eye can detect are red, green, and blue.
O True
O False
Answers
which is the best explanation for why organisms must meet their needs for resources?
a. So they can have more entertainment.
b. So they can survive and reproduce.
c. So they can continue sleeping all day.
d. So they can swim and fly faster.
Answers
Answer:
B
Explanation:
A isnt correct because animals have no emotions
C isnt correct because animals dont sleep all day
D isnt correct because not all organisms can swim or fly
B makes the most sense
What causes a ionic bond to occur
Answers
Answer:
when the valence (outermost) electrons of one atom are transferred permanently to another atom
Whay happens regularly in fission reaction
Answers
Answer:
In nuclear fission, an unstable atom splits into two or more smaller pieces that are more stable, and releases energy in the process. The fission process also releases extra neutrons, which can then split additional atoms, resulting in a chain reaction that releases a lot of energy.
Explanation:
State the coefficient required to correctly balance the following chemical equation:
_____ Ca3(PO4)2+ _____ NaCl ---------> _____ Na3PO4 + _____ CaCl2
Answers
Answer:
1 Ca3(PO4)2+ 6 NaCl ---------> 2 Na3PO4 + 3 CaCl2
Hydrofluoric acid and Water react to form fluoride anion and hydronium cation, like this HF(aq) + H_2O(l) rightarrow F(aq) + H_3O^+ (aq) At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition: Calculate the value of the equilibrium constant K_C for this reaction. Round your answer to 2 significant digits. K_C =
Answers
Answer:
Kc = 1.09x10⁻⁴
Explanation:
HF = 1.62g
H₂O = 516g
F⁻ = 0.163g
H₃O⁺ = 0.110g
To solve this question we need to find the moles of each reactant in order to solve the molar concentration of each reactan and replacing in the Kc expression. For the reaction, the Kc is:
Kc = [H₃O⁺] [F⁻] / [HF]
Because Kc is defined as the ratio between concentrations of products over reactants powered to its reaction coefficient. Pure liquids as water are not taken into account in Kc expression:
[H₃O⁺] = 0.110g * (1mol /19.01g) = 0.00579moles / 5.6L = 1.03x10⁻³M
[F⁻] = 0.163g * (1mol /19.0g) = 0.00858moles / 5.6L = 1.53x10⁻³M
[HF] = 1.62g * (1mol /20g) = 0.081moles / 5.6L = 0.0145M
Kc = [1.03x10⁻³M] [1.53x10⁻³M] / [0.0145M]
Kc = 1.09x10⁻⁴What is chemistry
What is the bond type in CaO
Answers
Answer:
Ionic bond
CaO is an ionic bond. Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. It is made up of one metal ion/cation(Ca^2+) and an non-metal ion/anion(O^2-).
whose model was discarded as a result of Rutherford's model?
A Dalton's model
B Thomson's model
C Bohr's model
D Quantum's model
Answers
Answer:
A. Dalton's model
Explanation:
Dalton's model was discarded as a result of Rutherford's model.
[tex]{ }[/tex]
[tex]\small\sf\:\:\:\:\:\:\:\:{:}\:\Longrightarrow{\bold{\pink{\sf{Thomson's \:model}}}}[/tex]
Rocks are classified as sedimentary, metamorphic, or igneous on the basis of
the
a. age of the rocks.
b. way the rocks were formed.
c. types of fossils the rocks contain.
d. number of minerals found in the rocks.
Answers
g The alkali metals are able to displace hydrogen readily from water. The alkaline earth metals are able to do so as well, although not nearly as vigorously. They are easily able to displace hydrogen from acid. a: Write and balance the equation for any of the alkali metals (pick your favorite!) reacting with water to form hydrogen gas and the metal's hydroxide (e.g. sodium hydroxide, potassium hydroxide). (10 points) b: Write and balance the equation for any of the alkaline earth metals (the second column on the left) reacting with hydrochloric acid to form hydrogen gas and the metal's chloride salt (e.g. magnesium chloride, calcium chloride) (10 points)
Answers
Answer:
See explanation
Explanation:
In writing a chemical reaction equation, we must ensure that it is balanced. In a balanced chemical reaction equation, the number of atoms of each element on the left hand side of the reaction equation must be equal to the number of atoms of the same atom on the right hand side of the reaction equation.
Let us now consider the reaction of NaOH with water as follows;
2Na(s) + 2H2O(l) ------> 2NaOH(aq) + H2(g)
For the reaction of Magnesium metal and HCl we have;
Mg(s) + 2HCl(aq) -------> MgCl2(aq) + H2(g)
what is the purpose of ash to soil conditioning.
Answers
Answer:
Question: what is the purpose of ash to soil conditioning?
answer: soil conditioner is a product that is added to soil to improve the soils physical qualities, it's usually the fertility of the soil and sometimes the mechanics of the soil. can be used to improve poor soils.
most wood ash contains a good percentage, about 25 percent, of calcium carbonate, which is an ingredient in garden lime. if your soil is highly acidic, with a pH of 5.5 or lower, amending with wood ash can raise the pH of your soil.
2Fe + 6HCl -> 2FeCl3 + 3H2 If 7.0 moles of HCl is added to enough iron that the HCl is completely used up, how many
moles of hydrogen gas will be produced?
Answers
Answer: 3.5 moles of hydrogen gas will be produced.
Explanation:
The balanced chemical equation is:
[tex]2Fe+6HCl\rightarrow 2FeCl_3+3H_2[/tex]
As HCl gets completely used up, [tex]HCl[/tex] is the limiting reagent.
According to stoichiometry :
6 moles of [tex]HCl[/tex] produces = 3 moles of [tex]H_2[/tex]
Thus 7.0 moles of [tex]HCl[/tex] produces=[tex]\frac{3}{6}\times 7.0=3.5moles[/tex] of [tex]H_2[/tex]
Thus 3.5 moles of hydrogen gas will be produced.
Scientists often use controlled experiments to answer questions. Choose ALL correct statements about controlled experiments. A) No changes are made to a test group in an experiment. B) A control group is used for comparison to other groups. C) Only one change can be made per test group in an experiment. D) Changes can only be made to the control group in an experiment. E) A controlled experiment must have a control group and test group(s).
Answers
Answer:
B) A control group is used for comparison to other groups.
E) A controlled experiment must have a control group and test group(s).
Explanation:
Answer:
B & E should be the answers
PLZ HELP URGENT!!!!!
Answers
Answer:
A fluke because it is an vertebrae
Explanation:
Identify the compound that contains an ionic bond.
*
O
H20
O CO2
O Naci
O CH3CH2OH
Answers
Answer:
Nacl compound contain ionic bonds because sodium is metal and chlorin is nonmetal
mathdrrggeszdrsz seer r-
Answers
Answer:
130!!
Explanation:
What is the molarity of a solution made by dissolving 1.25 mol of HCl in enough
water to make 625 mL of solution?
A) 0.073 M
B) 2.00 M
OC) 28.5 M
D) 500 M
Answers
Answer:
Explanation:
C
2.00 M is the molarity of a solution made by dissolving 1.25 mol of HCl in enough water to make 625 mL of solution.
What are moles?A mole is defined as 6.02214076 × of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
[tex]Molality = \frac{Moles \;solute}{Volume \;of \;solution \;in \;litre}[/tex]
[tex]Molality = \frac{1.25}{0.625}[/tex]
Molality = 2.00 M
Learn more about moles here:
brainly.com/question/8455949
#SPJ2
What rock forms from magma oozing onto the surface?
a. Igneous rock
b. Metamorphic rock
C. Sedimentary rock
d. Lava rock
Answers
CAN SOMEONE PLEASE HELP ME I WILL MARK AS BRAINLIEST :(
Answers
Answer:
ok so i did the problem and the answer was 8e+29
Explanation:
i dont really know what the answer is but thats the answer i got
sorry if this didnt help
Whoever gets these 2 right gets brainliest! :)
Answers
the first one is A covalent bond.
the second one is Answer: B Decomposition
PLEASE ANSWER!! THANK YA!
Using Graham's Law of Effusion, calculate
the approximate time it would take for
1.0 L of argon gas to effuse, if 1.0 L of
oxygen gas took 12.7 minutes to effuse
through the same opening.
Answers
Answer:
I think the answer is X
Explanation:
X is a variable and variables stand for the unknown
why blood is separated into different parts
Answers
Answer:
Blood fractionation is the process of fractionating whole blood, or separating it into its component parts. This is typically done by centrifuging the blood. The resulting components are: a clear solution of blood plasma in the upper phase (which can be separated into its own fractions, see Blood plasma fractionation),
Answer: Centrifugal force is used to separate the components of blood – red blood cells, platelets and plasma – from each other. ... The red blood cells precipitate to the bottom of the bag, with the platelets above them, then the white blood cells and the plasma at the very top. Also because Each part of the blood has a different function. Separating the blood into parts lets patients get only the specific part or parts of the blood that they need. So a whole blood donation can be used for several patients.
Hope this helps have a awesome day/nigh❤️✨t
Explanation:
What is the molar mass of V(CO3)2?
Answers
Just like the other person said ^, hope its right
Please need this ASAP. Calculate the mass of lime, CaO, that would be produced from 250 tonnes of limestone,
CaCO3.
Relative atomic masses: C 12; O 16; Ca 40.
Answers
Answer:
1.4×10⁸ g of CaO
Explanation:
We'll begin by converting 250 tonnes to grams (g). This can be obtained as follow:
1 tonne = 1×10⁶ g
Therefore,
250 tonne = 250 × 1×10⁶
250 tonne = 2.5×10⁸ g
Next, the balanced equation for the reaction.
CaCO₃ —> CaO + CO₂
Next, we shall determine the mass of CaCO₃ that decomposed and the mass CaO produced from the balanced equation. This can be obtained as follow:
Molar mass of CaCO₃ = 40 + 12 + (16×3)
= 40 + 12 + 48
= 100 g/mol
Mass of CaCO₃ from the balanced equation = 1 × 100 = 100 g
Molar mass of CaO = 40 + 16
= 56 g/mol
Mass of CaO from the balanced equation = 1 × 56 = 56 g
SUMMARY:
From the balanced equation above,
100 g of CaCO₃ decomposed to produce 56 g of CaO.
Finally, we shall determine the mass of CaO produced by the decomposition of 250 tonnes (i.e 2.5×10⁸ g) of CaCO₃. This can be obtained as follow:
From the balanced equation above,
100 g of CaCO₃ decomposed to produce 56 g of CaO.
Therefore, 2.5×10⁸ g of CaCO₃ will decompose to produce =
(2.5×10⁸ × 56)/100 = 1.4×10⁸ g of CaO.
Thus, 1.4×10⁸ g of CaO will be obtained from 250 tonnes (i.e 2.5×10⁸ g) of CaCO₃.
Mg(NO3)2 soluble or insoluble?
Answers
Answer:
The chemical compound Mg(NO3)2, also known as magnesium nitrate, is very soluble, especially in water.
After mixing for three hours, the product is extracted into dichloromethane and the solvent is removed to give 245 mg of an oil. Using the moles of our protected aldehyde calculated earlier (2.96) and the molecular weight of the product (102 g/mol) predict the theoretical 100% yield of the product in milligrams. Round to the tenths place.
Answers
Answer:
the theoretical 100% yield of the product in milligrams is 302920 mg
Explanation:
Given the data in the question;
245 mg of an oil
Using the moles of our protected aldehyde calculated earlier (2.96)
the molecular weight of the product (102 g/mol) = 102000 mg/mol
so, the mass of aldehyde produced (100% yield) will be;
⇒ number of moles × molar mass
⇒ 2.96 mol × 102000 mg/mol
⇒ 302920 mol.mg / mol
⇒ 302920 mg
Therefore, the theoretical 100% yield of the product in milligrams is 302920 mg