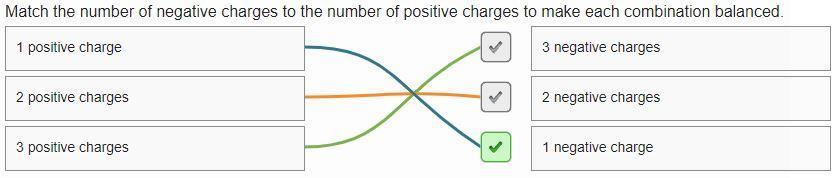
Answers
Answer : The correct match is:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Explanation :
As we now that there are three subatomic particles which are protons, electrons and neutrons.
The protons and neutrons are located inside the nucleus and electrons are located around the nucleus.
The protons are positively charged, the electrons are negatively charged and neutrons are neutral.
As we know that all the things are made up of charges and opposite charges attract to each other.
In a neutral atom, the positive charges and negative charges are balanced in an object. That means, in neutral atom the number of positive charges are equal to the negative charges.
So we can say that:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Answer:
1 positive---- 1 negative
2 positive---- 2 negative
3 positive---- 3 negative
Explanation:
edge 2023
Related Questions
To what volume would you need to dilute 200 mL of a 5.85M solution of Ca(OH)2 to make it a 1.95M solution?
Answers
Answer: 600 mL
Explanation:
Given that;
M₁ = 5.85 m
M₂ = 1.95 m
V₁ = 200 mL
V₂ = ?
Now from the dilution law;
M₁V₁ = M₂V₂
so we substitute
5.85 × 200 = 1.95 × V₂
1170 = 1.95V₂
V₂ = 1170 / 1.95
V₂ = 600 mL
Therefore final volume is 600 mL
what does celery, a wooden spoon, and oil/gasoline have in common?
Answers
Answer:
All of them are organic compounds which have carbon as their main atom in the structure.
Explanation:
Hello.
In this case, since organic chemistry is the study of all the compounds having the carbon atom as their main atom, all the vegetables, animals, an in general, living things are composed by lipids, proteins, and other organic substances with this feature. Moreover, wood-based materials are mainly composed by lignin which is an organic polymer also having carbon as the main atom. In addition, oil and gasoline are organic chemical compounds with a lot of applications in daily life which also contain carbon atoms in their structure.
In such a way, a celery, a wooden spoon, and oil/gasoline have the carbon atom in common as their main atom in their chemical structures.
Best regards.
what is the density of a block of wood measuring 9cmx2cmx6cm with the mass of 5.4g
Answers
Answer:
0.05 g/cm^3.
Explanation:
The volume of the block = 9*2*6 = 108 cm^3.
Density = mass/volume
= 5.4 / 108
= 0.05 g/cm^3.
HELPP
describe what potassium would do to be more stable
Answers
Answer:
Explanation:
Its a elemental potassium is soft ,white in colour and has one more electron than argon,an element that we know is extremely stable ... Potassium extra electron is easily lost to form the much more stable cation, K+
(6 points) Calculate the maximum number of moles and grams of H2S that can form when 158 g of aluminum sulfide reacts with 131 g of water: Show work for full poi
Answers
Answer:107.1 g, 124.1 g
Explanation:
The equation of the reaction is;
Al2S3(s) + 6H20(l) ----> 2Al(OH)3(s) + 3H2S(g)
Hence;
For Al2S3
Number of moles= reacting mass/molar mass
Number of moles = 158g/150gmol-1 =1.05 moles
If 1 mole of Al2S3 yields 3 moles of H2S
1.05 moles of Al2S will yield
1.05 × 3/1 = 3.15 moles
Mass of H2S = 3.15moles × 34 gmol-1 = 107.1 g
For water
Number of moles of water = 131g/18gmol-1= 7.3 moles
6 moles of water yields 3 moles of H2S
7.3 moles of water will yield 7.3 × 3/6 = 3.65 moles of H2S
3.65 moles × 34 gmol-1 =124.1 g
where are electrons found in an atom
Answers
Answer:
The electrons are found on the outer shell of the atom.
Explanation:
To vaporize/condense a substance, does the substance have to absorb or release heat?
Answers
Answer: it would release heat because the thermal energy it absorbed to become a gas. so it would release heat. hope this helps :)
Explanation:
PLEASE PLEAS HELP Which of the following compounds is insoluble in water?
a) ZnSO4
b) K2SO4
c) Na2CO3
d) Ag2CO3
Answers
Answer:
Your answer is d
Explanation: silver carbonate Ag2CO3 is insoluble in water
What occurs after cytokinesis is completed at the end of meiosis I?
O Four haploid cells are formed.
O Two diploid cells are formed.
OTwo haploid cells are formed.
O Four diploid cells are formed.
Answers
Answer. After cytokinesis is completed at end of meiosis - I two haploid cells are formed.on:
Answer:
C. TWO HAPLOID CELLS ARE FORMED
Explanation:
I TOOK THE EDGUNITY TEST AND I GOT IT CORRECT
will henry's law not be applicable if the solubility of the gas is very high
Answers
Answer:
No
Explanation:
Henry’s law is a gas law which states that at the amount of gas that is dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid when the temperature is kept constant. The constant of proportionality for this relationship is called Henry’s law constant (usually denoted by ‘kH‘). The mathematical formula of Henry’s law is given by:
P ∝ C (or) P = kH.C
Where,‘
P’ denotes the partial pressure of the gas in the atmosphere above the liquid.‘C’ denotes the concentration of the dissolved gas.‘kH’ is the Henry’s law constant of the gas.Limitations of Henry’s Law
This law is only applicable when the molecules of the system are in a state of equilibrium.Henry’s law does not hold true when gases are placed under extremely high pressure.The law is not applicable when the gas and the solution participate in chemical reactions with each other.Label each of the following changes as a physical change or chemical change. Give evidence to support your answer.
A catalytic converter changes nitrogen dioxide to nitrogen gas and oxygen gas
Answers
Answer:
A catalytic converter changes nitrogen dioxide to nitrogen gas and oxygen gas is a chemical change.
Explanation:
Hello.
In this case, since physical changes do not modify the molecular composition and structure of the material undergoing it whereas the chemical change does, for catalytic converters we should know they promote chemical reaction in which the composition is changed; for instance, for the given example, the following chemical reaction is the evidence:
[tex]2NO_2\rightarrow N_2+O_2[/tex]
As you can see, nitrogen and oxygen are no longer bonded but separated by themselves, therefore, this is a chemical change.
Best regards.
How many grams of H2SO4 are needed to prepare 5.0 L of a 2 M H2SO4 solution? You must show your work in order to receive credit.
Answers
To prepare 5L of a 2.0M solution you require: 98g/mol * 5mol * 2mol /L = 980g H2SO4
13. (6C) An unknown chemical has the
following properties: it is a white crystal,
reacts with water, and has a high boiling
point. Which of these properties is
physical?
A White color only
B Reacts with water, high
boiling point
C Reacts with water only
D White color, high boiling
point
Answers
Answer: it is B
Explanation:
Calculate the mass in 0.523 moles of Ag?
Answers
Answer:
56.41 g
Explanation:
Moles = Mass ÷ [tex]A_{r}[/tex]
Given that,
moles = 0.523 mol
[tex]A_{r}[/tex] = 107.8682 u
mass = ?
Mass = Moles × [tex]A_{r}[/tex]
Mass = 0.523 × 107.8682
mass = 56.41 g
A solution has a pH of 6. What is true about the solution?
A. It is a strong basic solution.
B. It is a weak acidic solution.
C. It is a weak basic solution.
D. It is a strong acidic solution.
please help me
Answers
Answer:
A. it is a strong basic solution
Answer:
(see below)
Explanation:
First, refer to the pH scale:
1 2 3 4 5 6 7 8 9 10 11 12 13 14
<== acidic neutral basic ==>
You can see that the smaller the number, the stronger the acid and the bigger the number, the more basic the base is. 7 is neutral, such as water. it's neither basic nor acidic.
Now, using the process of elimination:
A) It's a strong basic solution.
No, because this solution's pH hasn't even reached basic.
B) It's a weak acidic solution.
Yes, because it is acidic and it's just a little bit more acidic than a neutral solution.
C) It's a weak basic solution.
No, because this solution's pH hasn't even reached basic.
D) It's a strong acidic solution.
No, because even though it's acidic, it's just below neutral. For something to be a strong acidic solution would be around a pH of 3.
So the answer would be B) It's a weak acidic solution.
how can you tell where sugar enters the blood stream
please help asap
Answers
Answer:
Sugar can't enter cells directly , so when blood sugar level rises, ... signal for the release of insulin into the bloodstream.
Explanation:
yes when the sugar enter the bloodstream it may slowly slowly effect in your body and it may causes diabetes
You will need to balance this reaction
Na3PO4 +
CaCl2 → ? NaCl +_ Ca3(PO4)2
Answers
Answer:
2Na₃PO₄ + 3CaCl₂ → 6NaCl + Ca₃(PO₄)₂
Explanation:
Chemical equation:
Na₃PO₄ + CaCl₂ → NaCl + Ca₃(PO₄)₂
Balance chemical equation:
2Na₃PO₄ + 3CaCl₂ → 6NaCl + Ca₃(PO₄)₂
First step:
Na₃PO₄ + CaCl₂ → NaCl + Ca₃(PO₄)₂
Left hand side Right hand side
Na = 3 Na =1
P = 1 P = 2
O = 4 O = 8
Ca = 1 Ca = 3
Cl =2 Cl = 1
2nd step:
2Na₃PO₄ + CaCl₂ → NaCl + Ca₃(PO₄)₂
Left hand side Right hand side
Na = 6 Na =1
P = 2 P = 2
O = 8 O = 8
Ca = 1 Ca = 3
Cl =2 Cl = 1
3rd step:
2Na₃PO₄ + 3CaCl₂ → NaCl + Ca₃(PO₄)₂
Left hand side Right hand side
Na = 6 Na =1
P = 2 P = 2
O = 8 O = 8
Ca = 3 Ca = 3
Cl = 6 Cl = 1
3rd step:
2Na₃PO₄ + 3CaCl₂ → 6NaCl + Ca₃(PO₄)₂
Left hand side Right hand side
Na = 6 Na =6
P = 2 P = 2
O = 8 O = 8
Ca = 3 Ca = 3
Cl = 6 Cl = 6
If 0.4743 moles of H2O are produced, how many grams of VOCl3 will also be produced?
(V2O5 + 6 HCl → 2 VOCl3 + 3 H2O)
Answers
Answer:
i hope this helps. sorry if it totally doesn't
Use the image above to answer the
question.
1. (6B) What two particles are found in the
nucleus of an atom? (choose two options)
Protons
Neutrons
Nucleus
Electron
Electron cloud
Answers
Answer:
A-protons, B-nucleus, D-electrons, C-nucleus, E-electric cloud
Explanation:
What type of structural isomerism is shown by [Cr(NH3)4Cl2]Br and
[Cr(NH3)4ClBr]Cl?
Answers
Explanation:
Ionisation isomerism is shown by [Cr(NH₃)₄Cl₂]Br and [Cr(NH₃)₄ClBr]Cl
What is the density of a block of gold that occupies 1000 ml and has a mass of 3.5 kg? Show your work
Answers
Answer:
We are given:
mass of the block = 3500 grams
volume of the block = 1000 mL
Finding the density:
Density = mass of the object (in grams) / volume of the object (in mL)
Density = 3500 / 1000
Density = 3.5 g / mL
Helpppp!!!!! Please help me
Answers
Answer:
10.)Weight and mass are different because only weight describes the force of gravity on an object.
11.)On a planet with more mass than earth your weight would be higher because greater gravitational pull.
On a planet with less mass than earth your weight would be less because of the lower gravitational pull.
Explanation:
These are two metamorphic rocks.
Left: red and white rock with rounded grains and coarse texture. Right: flat, gray rock composed of thin layers.
Which statement about the rocks is accurate?
The rock on the right is foliated.
The rock on the left formed from granite.
The rock on the left is formed from cooled magma.
The rock on the right has randomly arranged grains.
Answers
Answer:
A. the rock on the right is foliated
Explanation:
because if you look at the image then you see that the rock is arranged in layers so you can see that it is a foliated rock and also I got it right on my quiz
HOPE IT HELPS!!!
What do you learned about homogeneous mixture?
Answers
Answer:
homogeneous is consisting a part of or people are similar to each other or are if same type.
When 28.0 g of acetylene reacts with hydrogen, 24.5 g of ethane is produced. What is the percent yield of C2H6 for the reaction?
C2H2(g) + 2H2(g) → C2H6(g)
Answers
Answer:
[tex]Y=75.6\%[/tex]
Explanation:
Hello.
In this case, since no information about the reacting hydrogen is given, we can assume that it completely react with the 28.0 g of acetylene to yield ethane. In such a way, via the 1:1 mole ratio between acetylene (molar mass = 26 g/mol) and ethane (molar mass = 30 g/mol), we compute the yielded grams, or the theoretical yield of ethane as shown below:
[tex]m_{C_2H_6}^{theoretical}=28.0gC_2H_2*\frac{1molC_2H_2}{26gC_2H_2}*\frac{1molC_2H_6}{1molC_2H_2} *\frac{30gC_2H_6}{1molC_2H_6}\\ \\m_{C_2H_6}^{theoretical}=32.3gC_2H_6[/tex]
Hence, by knowing that the percent yield is computed via the actual yield (24.5 g) over the theoretical yield, we obtain:
[tex]Y=\frac{24.5g}{32.3g}*100\%\\ \\Y=75.6\%[/tex]
Best regards.
molecular formula of C4H5
Answers
Answer:
Cyclopropylmethylene
A stream of oxygen enters a compressor at 298 K and 1.00 atm at a rate of 127m3/h and is compressed to 358 K and 1000 atm. Estimate the volumetric flow rate of compressed O2 using the compressibility-factor equation of state.
Answers
Answer:
The value is [tex]V_2 = 0.246 \ m^3/h[/tex]
Explanation:
From the question we are told that
The temperature at which the gas enters the compressor is
[tex]T_i = 298 \ K[/tex]
The pressure at which the gas enters the compressor is
[tex]P_I = 1.0 \ atm[/tex]
The volumetric rate at which the gas enters the compressor is
[tex]V = 127 m^3/h[/tex]
The temperature to which the gas is compressed to is
[tex]T_f = 358 \ K[/tex]
The pressure to which the gas is compressed to is
[tex]P_f= 1000 \ atm[/tex]
Generally the volumetric flow rate of compressed oxygen is evaluated from the compressibility-factor equation of state as
[tex]V_2 = V_1 *\frac{z_2}{z_1} * \frac{T_2}{T_1} * \frac{P_1}{P_2}[/tex]
Here [tex]z_1[/tex] is the inflow compressibility factor with value [tex]z_1 = 1[/tex]
Here [tex]z_1[/tex] is the outflow compressibility factor with value [tex]z_2 = 1.61[/tex]
So
[tex]V_2 = 127*\frac{1.61}{1} * \frac{358}{298} * \frac{1}{1000}[/tex]
[tex]V_2 = 0.246 \ m^3/h[/tex]
Select the term that matches each definition:
a) A decrease in the solubility of an ionic compound as a result of the addition of a common ion.
b) The mass of a salt in grams that will dissolve in 100 mL of water.
c) A solution that has dissolved the maximum amount of a compound at a given temperature. Any further addition of salt will remain undissolved.
d) The product of the molarities of the dissolved ions, raised to a power equal to the ion's coefficient in the balanced chemical equation.
e) The maximum number of moles of a salt that will dissolve in 1 L of solution.
*** Answer options for all questions: ***
- Solubility
- Molar Solubility
- Solubility product constant
- Common ion effect
- Saturated Solution
Answers
Answer:
a) common ion effect
b) solubility
c) saturated solution
d) solubility product constant
e) molar solubility
Explanation:
When a substance, say BA2 is dissolved in a solution and another substance CA2 is dissolved in the same solution. The solubility of BA2 is decreased due to the addition of CA2. This is known as common ion effect.
The mass of a substance that will dissolve in a given Volume of solvent is known as it's solubility.
The molar solubility is the amount of moles of solvent that dissolves in 1 dm^3 of solvent.
A solution that contains just as much solute as it can normally hold at a given temperature is known as a saturated solution.
Lastly, the product of molar solubilites raised to the power of the molar coefficient is know as the solubility product constant.
The correct matches and their explanation are:
a) A decrease in the solubility of an ionic compound as a result of the addition of a common ion: Option 4. common ion effect
It relates to the equilibrium effect that occurs due to the addition of common ions.b) The mass of salt in grams that will dissolve in 100 mL of water: Option 1. solubility
Solubility is the property of solute to form a solution with the solvent of another substance.c) A solution that has dissolved the maximum amount of a compound at a given temperature. Any further addition of salt will remain undissolved: Option 5. saturated solution
When the solution cannot accommodate any more addition of solute of a substance is called a saturated solution.d) The product of the molarities of the dissolved ions, raised to a power equal to the ion's coefficient in the balanced chemical equation: Option 3. solubility product constant
It is an equilibrium constant for the solid solute dissolved in the solution.e) The maximum number of moles of a salt that will dissolve in 1 L of solution: Option 2. molar solubility
Before the saturation of a solution, the amount of solute it can dissolve is called molar solubility.To learn more about molar solubility and common ion effect follow the link:
https://brainly.com/question/14782973
Give Me An Atom With The Following Characteristics Lanthanide series
Answers
A student asks why the ashes from a fire have a much lower mass than the wood that was burned.
Which is the correct answer to the student’s question?
Gases are released into the air.
Atoms in the wood are destroyed.
Heat causes the molecules to change color.
Water inside the wood solidifies.
(I am not in college)