Answers
Answer:
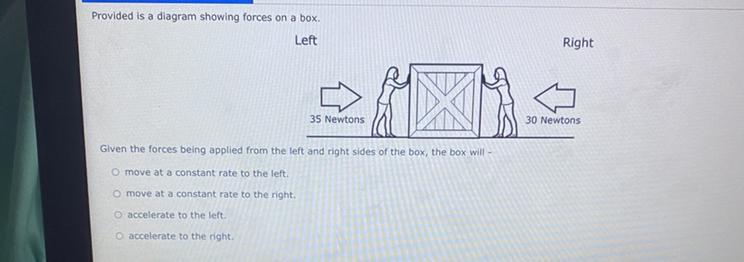
im pretty sure its move at a constant rate to the right
Related Questions
Which electron configuration represents the electrons in an atom of sulfur in an excited state? 2 – 8 – 6
2 – 7 – 7
2 – 8 – 7
2 – 7 – 8
Answers
The relations of the quantum numbers allow to find that the correct answer for the configuration of the excited state is:
2 - 7 - 7
The electronic configuration of the elements is the distribution of electrons and different levels and sublevels fulfilling the relationships between quantum numbers.
The principal quantum number (n) goes from 0 to infinity The orbital quantum number (l) goes from 0 to n-1, in general it is represented by lera s, p, d, f The magnetic quantum number ([tex]m_l[/tex]) ranges from -l to l The spin quantum number ([tex]m_s[/tex]) can have two values + ½ and - ½
In the base configuration Sulfur of the periodic table is 2 - 8 - 6
This is at the lowest energy configuration, when the atom acquires energy, an electron from its shell must be promoted to the next level.
That is, an electron from level n = 2 that is full is promoted to level n = 3 since in this shell there is still room for two electrons, the configuration of the excited state is:
2- 7 - 7
Let's examine the different alternatives:
1) 2 - 8 -6
False, this is the configuration of the base state
2) 2 - 7 - 7
True. An electron is promoted to the next level due to the excitation of the atom
3) 2 - 8 -7
False, in this configuration the atom becomes an ion
4) 2 - 7 - 8
False, there is an electron transfer between two levels, but there is an extra electron that turns that atom into an ion
In conclusion using the relationships of quantum numbers we can find that the correct answer for the excited state configuration is:
2 - 7 - 7Learn more here: brainly.com/question/16762037
The diagram shows a model of an animal cell. Explain how you'd modify the model to show the structures in a plant cell.
Answers
Answer:to show the structures of a plant cell,add a cell wall around the membrane and increase the size of the vaculoe insidethe cell, draw green ovals to represent chloroplasts.
Explanation:
Volcanic belts form along
a.
islands in the Pacific Ocean.
b.
North American mountain ranges.
c.
the boundaries of Earth’s plates.
d.
the coast of Antarctica.
Please select the best answer from the choices provided
Answers
Which statements about scientific explanations are true?
I. Scientific explanations must prove that a hypothesis is true.
II. Scientific explanations should be based on evidence or data.
III. Scientific explanations must have logical and consistent arguments.
IV. Scientific explanations should use scientific principles, models, and theories.
Answers
Answer:
The statement that is true is:
II. Scientific explanations should be based on evidence or data.
~Lylliara Jackson~
list the elements symbols for the elements that are designated with neon green
Answers
H, C, N, O, P, S, Se
I saw this on a worksheet hope this helps!
Answer:
h,c,n,o,p those are the elements
Do you think your meal would allow your cells to sufficiently build your cell
membranes? Why or why not?
Answers
Answer:
Cells are the fundamental units of life ' the bricks from which all your. which are built of your cells, will become compromised, and you can. of these new cells from the nutrients you get in your food is one way. Let's take a look inside one of your cells and see what the nutrients really do.
Explanation:
Which of the following best describes how Thomson
concluded that electrons are present in all atoms.
a
The charge-to-mass ratio of electrons is always the
same constant, no matter which substances are
used in the cathode ray tube.
b The cathode ray was able to turn a wheel.
The cathode ray always bent toward a negatively
charged plate.
d
The charge-to-mass ratio is unique to each different
element.
Answers
Answer: A
Explanation:
The charge-to-mass ratio of electrons is always the same constant, no matter which substances are used in the cathode ray tube.
Explanation:
Thomson in his model of atom discussed that the atom consists of a negative charge particle termed electron randomly distributed in the positively charged sphere to balance the negative charge.His model of the atom was also known as the Plum pudding model of the atom. In which electrons are embedded in positive soup.He discovered electrons by conducting an experiment with cathode rays in which cathode rays emerging from the cathode were observed to be deflected towards the positively charged plate.He also conducted the same experiment with different metals (for anode and cathode) and gases and found out that the charge to mass ratio of the electron was regardless of the metals or gases used in the experiment.With this, he landed on the conclusion that these particles of cathode rays are the universal component of matter.So, from this, we can conclude that the charge-to-mass ratio of electrons is always the same constant, no matter which substances are used in the cathode ray tube describes Thomson's conclusion that electrons are present in all atoms.
Learn more about J.J. Thomson's model of atom here:
brainly.com/question/2437167?referrer=searchResults
brainly.com/question/1874920?referrer=searchResults
32 N of Force. 37 N force. How strong is the net force and in which direction?
Answers
Explanation:
They are working against each other. That means you subtract.
32 - 37 = -5
You are moving 5 units to the left because 37 is negative which means it is moving left. The key word is left. In physics, you would get the same answer if you did it this way.
37 - 32 = 5
The 5 moves in the same direction as the 37
how many atoms can you fit on the head of a pin
Answers
Answer:
According to google, "About five million million hydrogen atoms could fit. Some factors would affect that number like the area of the head and the size of atoms (as well as attractions between atoms). Some atoms are larger than others." Is this accurate? I'm not sure. Good luck! :)
1 pc
Calculate the volume of a an object that has a height of 14.3cm, length or
25.2cm, width of 6.7cm. *
46.2cm
2414.412 cm
0.085 cm
53.785 cm
Answers
Answer:0.085
Explanation:
is ice forming and then melting back into water a physical change
Answers
yes it isssss abcdefghijk
Protons have a __________________ charge and are found in the area of an atom called ________________________.
Answers
Answer:
Positive
Nucleus
Water can undergo a change of state when its energy changes at specific temperatures. Solid water, or ice, changes into liquid water when its energy increases at its melting point. a. Describe this process. b. Explain how the energy changes with each state.
Answers
Answer: The solid turns into liquid during melting.
Explanation:
a. The process of a solid turning into liquid is called melting. The solid ice turns into a liquid during the melting process. The solid molecules gain energy and flow as a liquid.
b. The energy of the molecules increases in the liquid state. When the ice turns into a liquid the energy of the molecules increases. The application of heat or excess temperature increases the energy of the molecules.
This picture shows a model if a cell. What is the main function of the part labeled Z in the model?
Answers
Answer:
store water so b
Explanation:
labeled Z in the model refers to store food and other material in the cell.
what is cell?
cell can be defined as the basic unit of the living cell, it can be unicellular or multicellular. unicellular organism are prokaryote and some of the unicellular eukaryotes whereas multicellular organisms are eukaryotes like plants and animals.
a basic component of cell are cell membrane, cytoplasm, nucleus, mitochondria, lysosome, golgi body, endoplasmic reticulum.
cell wall only present in plant cell and prokaryotic cell while it is absent in animal cell, the function of cell wall is to provide protection
For more details cell, visit
https://brainly.com/question/3142913
#SPJ5
How is a substance’s solubility in another substance determined?
A. Add solvent till no more will dissolve
B. Add solvent stepwise till no more will dissolve
C. Add solute until no more will dissolve in a fixed amount of solvent
D. Cool the solvent
Answers
Answer:
b
Explanation:
now explanation because that is answer
why does the moon shine
Answers
Answer:
The moon reflects light from the sun making it appear bright in the sky.
Explanation:
Answer: Its a reflection of the sun's light
Explanation: the light from the sun bounces off of the moon
If water does break down by electrolysis to form new substances, what might be formed?
Answers
Why are the coefficients before a chemical symbol important?F. They show the number of elements in each molecule.G. They indicate the number of molecules of each substance.H. They describe the number of times each molecule reacts.J. They explain the order in which the reaction takes place
Answers
Answer:They indicate the number of molecules of each substance
Explanation:
When we write chemical reaction equations, we usually put a number before the symbol of each reactant.
These coefficient shows the number of molecules of that specie that is involved in the balanced reaction equation for the reaction.
These coefficients are important because they are used in carrying out stoichiometric calculations.
If an object has a mass of 180 g and a density of 6 g/cm^3, what is the volume of the object?
Answers
Answer:
The answer is 30 cm³Explanation:
The volume of a substance when given the density and mass can be found by using the formula
[tex]volume = \frac{mass}{density} \\[/tex]
From the question
mass = 180 g
density = 6 g/cm³
We have
[tex]volume = \frac{180}{6} \\ [/tex]
We have the final answer as
30 cm³Hope this helps you
Explain how you would dilute a 1 mol/dm solution to one-tenth of its original
concentration.
Answers
Answer:
13.123.
Explanation:
How many protons, electrons and neutrons do A nitrogen atom with atomic number 7 and mass number 14
Answers
Answer:
p =7
e = 7
n = 7
Explanation:
proton = atom number
electron = atom number - muatan
neutron = mass nimber - atomic number
What metal is Potassium (K)?
Answers
Explanation:
Potassium is a chemical element with the symbol K and atomic number 19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure
Answer:
alkali metalExplanation:
Potassium is a chemical element with symbol K and atomic number 19. Classified as an alkali metalWhich particle is NOT made up of quarks?
A: a proton
B: a neutron
C: an electron
Answers
Answer: C: an electron
Explanation:
Answer:
C: electrons
Explanation:
What is the mass of 4.49 x 10º carbon dioxide, CO2, molecules?
Answers
4.49 x 10^0 / 44 g/mol = .1020 mol CO2
1 mol = 6.022 x 10^23 molecules (avogadros number)
so .1020 mol x 6.022 x 10^23 =
6.14 x 10 ^22 molecules CO2
Is this actually right
Answers
Answer:
what?????????i don't see a question
Answer:
Yes
Explanation:
What is an atomic theory?
What is an isotope?
How is the atomic mass of an element calculated?
Answers
Answer:
Atomic theory is the scientific theory that matter is composed of particles called atoms.
isotopes are variants of a particular chemical element which differ in neutron number, and consequently in nucleon number.
to calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons.
honey please click the thanks button
thanks babe!
Explanation:
Which of the following is true about pure substances? A. A pure substance has the same chemical properties throughout. All pure substances are unable to be separated by any means. c. A pure substance can only be made up of one kind of atom. D. A pure substance can always be separated by physical means.
Answers
Answer:first one
Explanation:
A pure substance has same chemical properties throughout , it cannot be separated by any means and it is made up of one kind of atom.
What are chemical properties?
These properties are defined as those properties which become evident during or after a chemical reaction after the identity of the substance is changed during chemical reaction.
These properties cannot be determined externally just by viewing the substance ,these change immensely after a substance undergoes a chemical change.These are used for identification of unknown substances and for building up chemical classifications.
The major chemical properties are flammability,toxicity,reactivity,acidity and heat of combustion.For a chemical property to be apparent , it is necessary that the structure of the substance is altered.
Learn more about chemical properties,here:
https://brainly.com/question/1935242
#SPJ2
ultraviolet radiation has a higher frequency than visible light. which type of light wave carries more energy?
a=they carry the same amount of energy
b=visible light
c= ultraviolet radiation
Answers
Answer:
The answer to this question is C; ultraviolet radiation
Explanation:
The the reason for this is because it carries more energy per photon than visible light does. Light travels at a speed of 299,792 kilometers per second (about 186,282 miles per second).
Where do rivers usually begin?
at the ocean
at groundwater outlets
in mountainous regions
in lakes and larger rivers
Answers
Answer:
in mountainous regions
Explanation:
The rivers usually begins in mountainous regions. A river's source is typically located in a high area, such a mountain or a hill. A river may have several sources. Some rivers start where an underground natural spring discharges water.
What is a mountain ?A mountain is an elevated area of the crust of the Earth, typically with steep sides that reveal a substantial amount of bedrock that has been exposed.
Although definitions vary, a mountain is often taller than a hill, rising normally at least 300 meters above the surrounding terrain, and differs from a plateau in having a constrained peak region.
Every river has a beginning place where the water flow starts. We refer to this source as a headwater. Some rivers have their beginnings in hills or mountains, where rainwater or snowmelt gathers and creates little channels.
It is possible for the headwater to originate from mountain snowmelt or rainfall, but it is also possible for it to bubble up from the ground or develop at the edge of a lake or big pond.
Thus, option C is correct.
To learn more about mountain, follow the link;
https://brainly.com/question/10690247
#SPJ6
In Ernest Rutherford's gold foil experiments, some alpha particles were deflected from their original paths, but most passed through the foil with no deflection. Which statement about gold atoms is supported by these experimental observations?
Gold atoms consist mostly of empty space.
Gold atoms are similar to alpha particles.
Alpha particles and gold nuclei have opposite charges.
Alpha particles are less dense than gold atoms.
Answers
Answer:
A
Explanation: