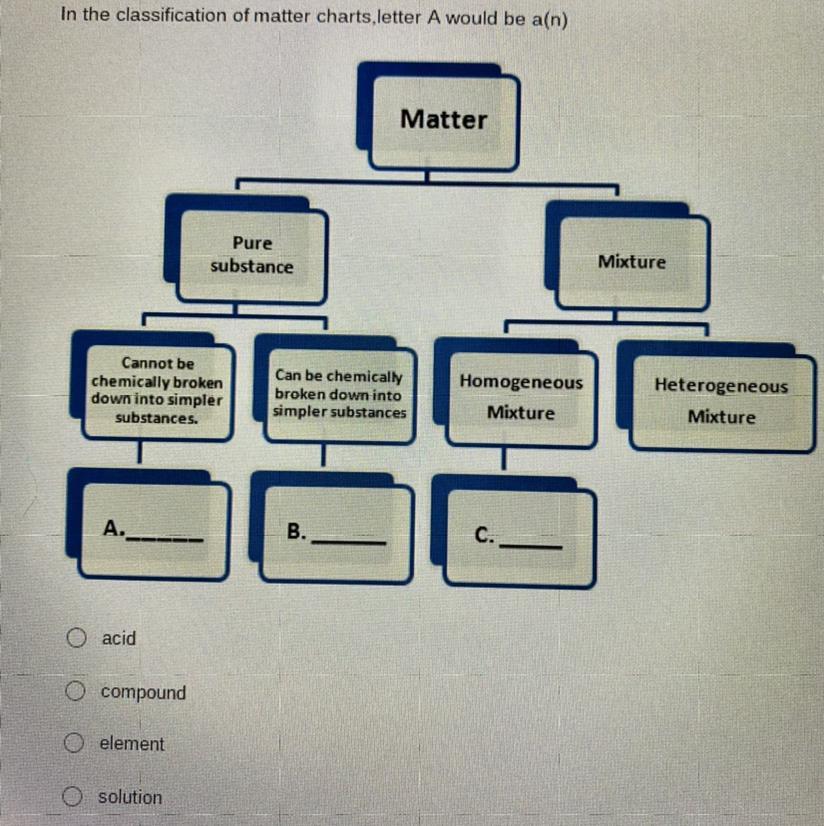
Answers
Answer:
A=element
B=compond
C=solution
Related Questions
The lewis dot notation for two atoms is shown. What is represented by this notation? K loses one portion to CI, K gains one portion from CI, K loses one electron to CI, K gains one electron from CI
Answers
Answer:
K loses one electron to CI
Explanation:
The lewis electron dot notation shows only the chemical symbol of the element surrounded by dots to represent the valence electrons.
We have atom of K with one valence electrons
Cl with 7 valence electrons
For an electrostatic attraction to occur, both particles must be charged. To do this, one of the species must lose an electron, and the other gains it.
This will make both species attain a stable octet;
Hence, K will lose 1 electron and Cl will gain the electrons.
Answer:
C: K loses one electron to CI
Explanation:
I took the test and got it correct!!
determine the total amount of heat, in joules, required to completely vaporize a 50.0 gram sample of H20 at its boiling point at standard pressure
Answers
Answer: 1.13 x 10^5
Explanation:
Please help me!!!:)))
Answers
Answer:
blocks 1 and 2 the rhdh huff hgfhh5
Element
Molar mass (g/mol)
1.008
H
С
12.01
16.00
Using the information in the table, calculate the number of moles in a 2.03 kg sample of citric acid
(C6H8O7)
Write your answer using three significant figures.
mol C6H8O7-
Answers
Answer:
Number of moles = 10.6 mol
Explanation:
Given data:
Molar mass of H = 1.008 g/mol
Molar mass of C = 12.01 g/mol
Molar mass of O = 16.00 g/mol
Mass of citric acid = 2.03 kg (2.03×1000 = 2030 g)
Number of moles of citric acid = ?
Solution:
Formula:
Number of moles = mass/molar mass
Now we will calculate the molar mass of citric acid:
C₆H₈O₇ = (12.01× 6) + (1.008×8) + (16.00×7)
C₆H₈O₇ = 72.06 + 8.064+112
C₆H₈O₇ = 192.124g/mol
Number of moles = 2030 g/ 192.124g/mol
Number of moles = 10.6 mol
The number of moles in a 2.03 kg sample of citric acid is :
- 10.6 mol
Molar MassGiven:
Molar mass of H = 1.008 g/mol
Molar mass of C = 12.01 g/mol
Molar mass of O = 16.00 g/mol
Mass of citric acid = 2.03 kg (2.03×1000 = 2030 g)
Number of moles of citric acid = ?
Formula:
Number of moles = mass/molar mass
The molar mass of citric acid:
C₆H₈O₇ = (12.01× 6) + (1.008×8) + (16.00×7)
C₆H₈O₇ = 72.06 + 8.064+112
C₆H₈O₇ = 192.124g/mol
Number of moles = 2030 g/ 192.124g/mol
Number of moles = 10.6 mol
Learn more about "Molar Mass":
https://brainly.com/question/12127540?referrer=searchResults
Choose all the answers that apply.
Fluorine (F) has seven electrons in its outermost shell. Fluorine _____.
-is a metal
-fills its shell by giving up seven electrons
-fills its shell by gaining one electron
-becomes a positively charged ion
-becomes a negatively charged ion
-is a nonmetal
Answers
Answer:
Fluorine is a non-metal, fills its shell by gaining one electron and becomes a negatively charged ion.
Explanation:
Fluorine have seven electrons in its outermost shell. This means that fluorine is located in Group 17(Halogens) in the Periodic Table. Note that halogens are non-metal elements. So fluorine is a non-metal.
Fluorine fills its shell by gaining one electron because it only needs one electron to achieve stable octet electron arrangement. ( it's easier to gain one electron than to lose all seven electrons in the outermost shell)
As fluorine atom gain one electron to achieve stable octet electron arrangement, its number of electrons becomes greater than its number of protons. So it becomes a negatively charged ion.
If the visible light spectrum is from 400 to 700 nm, would light with an energy of 2.79 x 10^-19 J be visible with the naked eye? What is the wavelength of this light?
Answers
Answer:
713 nm. It is not visible with the naked eye.
Explanation:
Step 1: Given data
Energy of light (E): 2.79 × 10⁻¹⁹ JPlanck's constant (h): 6.63 × 10⁻³⁴ J.sSpeed of light (c): 3.00 × 10⁸ m/sWavelength (λ): ?Step 2: Calculate the wavelength of the light
We will use the Planck-Einstein equation.
E = h × c / λ
λ = h × c / E
λ = 6.63 × 10⁻³⁴ J.s × 3.00 × 10⁸ m/s / 2.79 × 10⁻¹⁹ J
λ = 7.13 × 10⁻⁷ m
Step 3: Convert "λ" to nm
We will use the relationship 1 m = 10⁹ nm.
7.13 × 10⁻⁷ m × (10⁹ nm/1 m) = 713 nm
This light is not in the 400-700 nm interval so it is not visible with the naked eye.
Complete the reactions of Sn(II) and Sn(IV), and be sure that the reactions are balanced. Do not include the phases (liquid, aqueous, etc.). If no reaction occurs, leave the products side of the equation
completed reaction: SnBr2+PbBr4⟶
completed reaction: SnBr4+PbBr2⟶
Select the statements that are true about the reactions.
A. PbBr4 is more stable than PbBr2.
B. The inert‑pair effect renders Sn(II) as the more stable oxidation state of tin.
C. Sn(IV) is the most stable oxidation state of tin.
D. The inert‑pair effect renders Pb(II) as the more stable oxidation state of lead.
Answers
Answer:
The Inert Pair effect renders Pb(II) as the more stable oxidation state of lead
Explanation:
SnBr4 + PbBr2 ---> SnBr2 + PbBr4
SnBr2 + PbBr4 ---->
The Inert pair effect is mostly observed between group 15-17 in the periodic table. It leads to stability of the lower oxidation state of an element.
The reason for the Inert pair effect is that the s electrons become Inert due to poor shielding of the d and f-electrons. The Inert pair effect is a tendency of the s electrons not to participate in bonding (remain an Inert pair).
Owing to the Inert pair effect, Pb II is more stable than Pb IV
The smallest form of matter that still retains the properties of an element
Answers
Answer:
atom
Explanation:
the atom is the smallest form.
Item 4
Which statement is one of the three parts of cell theory?
Cell organelles can be membrane-bound or not membrane-bound.
Cells make up tissues, tissues make up organs, organs make up systems, systems make up organisms.
The smallest living things are single cells, and cells are the functional units of multicellular organisms.
There are three types of cells, prokaryotic, eukaryotic plant, and eukaryotic animal.
Answers
How many elements are in calcium dihydrogren phosphate
Answers
The energy associated with the motion and position of an object is called a.kinetic energy
b.potential energy
c.gravitational potential energy d.mechanical energy
Answers
Answer: The answer is D.
Explanation:
The form of energy associated with the motion, position, or shape of an object is called mechanical energy. An object's mechanical energy is a combination of its potential energy and its kinetic energy. The basketball has both potential energy and kinetic energy.
Hope this helps!
Name the following ionic compound: Ba(OH)2*2H2O
Answers
Metals typically lose electrons to complete their octet in a reaction with non-metals, whereas non-metals typically acquire electrons to complete their octet. Ionic compounds are typically formed via reactions between metals and nonmetals. The given compound barium hydroxide is ionic.
Ions with the opposite charge are carefully packed together to form crystalline solids. Ionic compounds typically result from reactions between metals and non-metals. The electrostatic interaction between the positive and negative ions holds ionic solids together.
Baryta, commonly known as barium hydroxide, has the chemical formula Ba(OH)₂. It is an odorless, clear-white powder. It has a toxic disposition. It is ionic in nature, with two hydroxide ions per molecule of barium hydroxide (Ba(OH)₂ in an aqueous solution an example.
To know more about ionic compound, visit;
https://brainly.com/question/13058663
#SPJ6
What is the density of a block of gold that occupies 1000 ml and has a mass of 3.5 kg? Show your work
Answers
Answer:
Density of block of gold is 3.5 g/cm³.
Explanation:
Given data:
Volume of block = 1000 mL
Mass of block = 3.5 kg (3.5×1000 = 3500 g)
Density of block = ?
Solution:
Density of substance is calculated by dividing the mass of substance over its volume.
Formula:
d = m/v
d = 3500 g/ 1000 mL
d = 3.5 g/mL
or
d = 3.5 g/cm³ (1ml = 1cm³)
Which of these is a source of pollution caused by humans?
A)
volcances
B)
pesticides
lightning strikes
D)
biological decay
Answers
Answer:
biological decay
Explanation:
Hope this helps
When solid Fe metal is put into an aqueous solution of SnSO4, solid Sn metal and a solution of FeSO4 result. Write the net ionic equation for the reaction.
Answers
Answer:Fe(s) + Sn^2+(aq) ----> Fe^2+(aq) + Sn(s)
Explanation:
The net ionic equation involved is;
Fe(s) + Sn^2+(aq) ----> Fe^2+(aq) + Sn(s)
We must recall that iron is above tin in the electrochemical series. The implication of this is that, iron has a more negative reduction potential compared to tin.
A metal can displace metals below it in the electrochemical series from their aqeous solution. Hence, iron displaces tin from it's solution.
Write both the complete electron-configuration notation and the noble-gas notation for a barium atom.
Answers
Answer:
Explanation:
Noble gas notation: [Xe] 6s2
Complete Electron Configuration: 1s22s22p63s23p63d104s24p64d105s25p66s2.
(ik kinda hard to understand but i looked it up ant it works)
Answer:
Complete Electronic Configuration:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²
Electronic Configuration in Noble Gas Notation:
[Xe] 6s²
Given the volume of a gas at 200mL at 1.05atm pressure, calculate the volume of the same gas at 1.01atm. The temperature is held constant.
Answers
Answer:
The new pressure will be
1000 L
, rounded to one significant figure.
Explanation:
Boyle's law states that when a gas is held at a constant temperature and mass in a closed container, the volume and pressure vary inversely. The equation to use is
P
1
V
1
=
P
2
V
2
.
Given
V
1
=
200
mL
×
1
L
1000
mL
=
0.2 L
P
1
=
700 mmHg
V
2
=
100
mL
×
1
L
1000
mL
=
0.1 L
Unknown
P
2
Equation
P
1
V
1
=
P
2
V
2
Solution
Rearrange the equation to isolate
P
2
and solve.
P
2
=
P
1
V
1
V
2
P
2
=
(
700
mmHg
×
0.2
L
)
0.1
L
=
1400 L
, which must be rounded to
1000 L
because all of the measurements have only one significant figure
Explanation:
What is a chelating agent?
Answers
Answer:
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents.
Answer:
Chemical compound that react with metal ions. They can form several covalent bonds to a metal without changing its own structure.
Explanation:
There's a list of chelating agents you can find in your book probably. An application of chelating agents is to transport metal ions across the membranes.
you should also know:
Chelating agents are any compound that reacts with a metal ion to produce a metal ion.
Ligand ion molecule or functional group that binds to another chemical to make a larger complex.
They are used to detoxify poisonous metal agents by binding and converting them so the body can excrete them without further harm.
Calculate the theoretical density (in g/cm3) of copper (Cu), given that it has the FCC structure. The atomic weight of Cu is 63.55 g/mol, and its atomic radius R is 0.1278 nm.
Answers
Answer:
8.937g/cm³
Explanation:
To answer this question we need to know that, in 1 unit FCC cell you have:
Edge length = √8 * R
Volume = 8√8 * R³
And there are 4 atoms per unit cell
Mass of 4 atoms in g:
4 atom * (1mol / 6.022x10²³atom) * (63.55g / mol) = 4.221x10⁻²²g
Volume in cm³:
0.1278nm * (1x10⁻⁷cm / 1nm) = 1.278x10⁻⁸cm
Volume = 8√8 * (1.278x10⁻⁸cm)³
Volume = 4.723x10⁻²³cm³
And density is:
4.221x10⁻²²g / 4.723x10⁻²³cm³ =
8.937g/cm³A substance that is dissolved in a solution is called a(n) __________________.
solute
compound
ion
Answers
Answer:
solute
Explanation:
Which statement best demonstrates how data from a global positioning system (GPS) can be used to lessen the effects of a
wildfire? (1 point)
GPS data can be used by people to quickly evacuate an area because of a wildfire
GPS data can be used by scientists to predict weather patterns that can lead to a wildfire
GPS data can be used by firefighters to identify the boundaries of a wildfire
GPS data can be used by first responders to calculate the safest route to a wildfire
Answers
Answer: here is your answer
Explanation: You are visiting your Grandmother and notice that she is eating a balanced diet, taking vitamins, getting the proper amount of sleep and is not overweight. Despite her healthy lifestyle, she appears run down and tired. You realize that it's due to her lack of physical activity. Write a convincing letter to your grandma explaining the benefits of participating in regular physical activity.
What processes are related to metamorphism
Answers
Answer:
Metamorphism is the change of minerals or geologic texture (distinct arrangement of minerals) in pre-existing rocks (protoliths), without the protolith melting into liquid magma (a solid-state change). The change occurs primarily due to heat, pressure, and the introduction of chemically active fluids.
Explanation:
i hope this helps :)
How many moles of precipitate will be formed when 100.0 mL of 0.200 M NaBr is reacted with excess Pb(NO₃)₂ in the following chemical reaction?
2 NaBr (aq) + Pb(NO₃)₂ (aq) → PbBr₂ (s) + 2 NaNO₃ (aq)
Answers
Answer : The number of moles of precipitate, [tex]PbBr_2[/tex] formed will be 0.01 moles.
Explanation : Given,
Concentration of NaBr = 0.200 M
Volume of solution = 100.0 mL = 0.1 L (1 L = 1000 mL)
First we have to calculate the moles of NaBr.
[tex]\text{Moles of NaBr}=\text{Concentration of NaBr}\times \text{Volume of solution in L}[/tex]
[tex]\text{Moles of NaBr}=0.200M\times 0.1L=0.02mol[/tex]
Now we have to calculate the moles of precipitate, [tex]PbBr_2[/tex] formed.
The balanced chemical reaction is:
[tex]2NaBr(aq)+Pb(NO_3)_2(aq)\rightarrow PbBr_2(s)+2NaNO_3(aq)[/tex]
From the balanced chemical reaction we conclude that:
As, 2 moles of NaBr react to give 1 mole of [tex]PbBr_2[/tex]
So, 0.02 moles of NaBr react to give [tex]\frac{0.02}{2}=0.01[/tex] mole of [tex]PbBr_2[/tex]
Therefore, the number of moles of precipitate, [tex]PbBr_2[/tex] formed will be 0.01 moles.
The number of mole of the precipitate (i.e PbBr₂) formed when 100 mL of 0.2 M NaBr react with excess Pb(NO₃)₂ is 0.01 mole
We'll begin by calculating the number of mole of NaBr in 100 mL of 0.2 M NaBr solution. This can be obtained as follow:Volume = 100 mL = 100 / 1000 = 0.1 L
Molarity of NaBr = 0.2 M
Mole of NaBr =?Mole = Molarity x Volume
Mole of NaBr = 0.2 × 0.1
Mole of NaBr = 0.02 mole Finally, we shall determine the number of mole of the precipitate (i.e PbBr₂) produced from the reaction. This can be obtained as follow:2NaBr(aq) + Pb(NO₃)₂(aq) → PbBr₂(s) + 2NaNO₃ (aq)
From the balanced equation above,
2 moles of NaBr reacted to produce 1 mole of PbBr₂.
Therefore,
0.02 mole of NaBr will react to produce = [tex]\frac{0.02}{2} \\\\[/tex] = 0.01 mole of PbBr₂.
Thus, the number of mole of the precipitate (i.e PbBr₂) produced from the reaction is 0.01 mole
Learn more: https://brainly.com/question/19572703
What is the pH of a bleach solution that has a [OH−]=1.3×10−4 M?
Answers
Answer:
pH = 10.113
Explanation:
Here, we can find pOH first:
pOH = [tex]-log([OH^-])[/tex] = 3.886.
Then, we can find pH which is 14 - pOH. We then get the answer above.
Then, we can find pH which is 14 - pOH. We then get the answer above.
The pH of acid is between 0-7 on pH scale while for base pH range is from 7-14. Thus the pH of 1.3×10⁻⁴ M bleach solution is 10.62.
What is pH?pH is a measurement of amount of hydronium ion H₃O⁺ in a given sample. More the value of hydronium ion concentration, more will be the solution acidic.
On subtracting pH from 14, we get pOH which measures the concentration of hydroxide ion in a given solution. pH depend on the temperature. At room temperature pH scale is between 0 to 14. pH of neutral solution is 7
The concentration of bleach solution is 1.3×10⁻⁴ M
Concentration of OH⁻=1.3×10⁻⁴ M
Mathematically,
pOH=-log[OH⁻]
Substituting the values
pH=-log[1.3×10⁻⁴]
= 3.886.
pH+ POH=14
pH=14-3.886.=10.62
Therefore, the pH of 1.3×10⁻⁴ M bleach solution is 10.62.
To learn more about pH, here:
https://brainly.com/question/27945512
#SPJ3
WHOEVER ANSWERS THIS GETS A SHOUTOUT ON INSTA LIKE I DONT EVEN CARE HELP
The heater used in a 4.33 m x 3.43 m x 3.03 m dorm room uses the combustion of natural gas (primarily methane gas) to produce the heat required to increase the temperature of the air in the dorm room. Assuming that all of the energy produced in the reaction goes towards heating only the air in the dorm room, calculate the mass of methane required to increase the temperature of the air by 7.35 °C. Assume that the specific heat of air is 30.0 J/K-mol and that 1.00 mol of air occupies 22.4 L at all temperatures. Enthalpy of formation values can be found in this table. Assume gaseous water is produced in the combustion of methane.
Answers
Answer:
The answer is 7.89
Explanation:
Mass of methane required to increase the temperature of the air in the room by 7.35 °C is 7.95 g
The volume of air in the room is first calculated:
Volume of air in the room = 4.33 m x 3.43 m x 3.03 = 45.00 m³
1 m³ = 1000 L
45.00 m³ = 45.00 m³ * 1000 L/m³
Volume of air in L = 45000 L
Number of moles of air in 45000 L of air is then determined:
1.00 moles of air occupies 22.4 L
number of moles of air in 45000 L = 45000 L * 1 mole / 22.4 L
number of moles of air = 2008.93 moles of air
Energy that is needed to heat the room by 7.35 °C is then calculated:
Quantity of energy needed = Specific heat capacity * number of moles * temperature increase
Specific heat capacity of air = 30.0 J/K/mole
Quantity of energy needed = 30.0 * 2008.93 * 7.35
Quantity of energy needed = 442969.065 J = 443.00 kJ
The amount of methane required to produce that amount of energy is then calculated:
Equation of combustion of methane : CH₄ + 2 O₂ ---> CO₂ + 2 H₂O
Enthalpy of combustion of methane = −890.3 kJ/mole
Number of moles of methane required = 443.00 kJ / 890.8 kJ/mole = 0.497 moles
Mass of 1 mole of methane = 16.0 g
mass of 0.497 moles of methane = 16.0 * 0.497 = 7.95 g
Therefore, mass of methane required to increase the temperature of the air in the room by 7.35 °C is 7.95 g
Learn more at: https: brainly.com/question/4213585
2-Methycyclohexanol is prepared commercially by catalytic hydrogenation of ocresol (2-methylphenol) and consists of a mixture of cis and trans isomers. (Note the spectrum is for the mixture.) (Hint: What do the peaks at 3.05 ppm and 3.75 ppm represent and what does their integration show
Answers
Answer:
Explanation:
From the information given :
The cis and trans isomer are in the ratio of 1:3, and the description of how the ratio of the cis- and trans-2-methylcyclohexanols from the 1H NMR spectrum can be explained as follows:
We are being told that the trans isomer's peak is 3.75 ppm (part per million). However, because the methyl group is far away from the OH group, it is less shilled than the cis isomer, which peaked at 3.05. Thus, the peak at 3.05 occurs in an area whose integration is three (3) times more than the peak at 3.75.
What types of materials are better at absorbing energy from radiation?
Answers
Answer:
Explanation:
Materials are clothes and heavy rope
Which is NOT an example of plants demonstrating the characteristics of life?
A. Plants are forces to make adaptations depending on its environment
B. A plant has stimuli that cause it to grow towards the sun
C. There are stages of growth that plants go through depending on favorable conditions
D. The leaves on a plant move in reaction to the wind
Answers
Answer:
D
Explanation:
trust me bro ive done this
Option D does not represent an example of plants demonstrating the characteristics of life.
The following information should be considered:
Plants to be treated as the forces for making the adaptions and based on the environment. The plant contains stimuli that result to grow towards the sun. There should be the growth stages based on the favorable conditions.Therefore we can conclude that Option D does not represent an example of plants demonstrating the characteristics of life.
Learn more: brainly.com/question/20934155
A teaspoon of salt, NaCl has a mass of about
5.0 g. How many formula units are in a
teaspoon of salt?
Answers
Answer: The answer is 5.15x10^22
Explanation:
The formula unit present in a teaspoon of salt [tex]NaCl[/tex] having a mass of about 5.0 g is [tex]5.15 \times10^{22}[/tex] formula units.
Molar mass, also known as molecular weight, is the mass of one mole of a substance. It is calculated by summing up the atomic masses of all the atoms in a molecule. The unit of molar mass is grams per mole (g/mol).
Now, to determine the number of formula units in a teaspoon of salt (NaCl), we need to use Avogadro's number and the molar mass of NaCl.
Avogadro's number [tex](N_a)[/tex] is approximately. [tex]6.022 \times10^{23}[/tex] formula units per mole.
The molar mass of [tex]NaCl[/tex] is the sum of the atomic masses of sodium (Na) and chlorine ([tex]Cl[/tex]), which are approximately 22.99 g/mol and 35.45 g/mol, respectively.
To calculate the number of formula units in 5.0 g of [tex]NaCl[/tex], we can follow these steps:
Now, calculate the number of moles of [tex]NaCl[/tex] using its molar mass:
Moles = Mass / Molar mass
Moles = [tex]5.0 g[/tex] / [tex](22.99 g/mol + 35.45 g/mol)[/tex]
Calculate the number of formula units using Avogadro's number:
Formula units = [tex]Moles \times Avogadro's number[/tex]
Let's perform the calculation:
Molar mass of [tex]NaCl[/tex]= [tex]22.99 g/mol + 35.45 g/mol = 58.44 g/mol[/tex]
Moles of [tex]NaCl[/tex] = [tex]5.0 g[/tex] / [tex]58.44 g/mol[/tex] ≈ [tex]0.0856 mol[/tex]
Formula units = [tex]0.0856 mol \times (6.022 \times 10^{23})[/tex] formula units/mol ≈ [tex]5.15 \times10^{22}[/tex]formula units.
Therefore, there are approximately [tex]5.15 \times10^{22}[/tex] formula units in a teaspoon of salt ([tex]NaCl[/tex]) having mass [tex]5.0 g[/tex].
Learn more molar mass about here:
https://brainly.com/question/31545539
#SPJ2
what are the strengths in the bonds of potassium bromide
Answers
Answer: Potassium Bromide (KBr) The Ionic bond formed between Potassium and Bromine is created through the transfer of electrons from Potassium (metal) to Bromine (nonmetal).
Explanation: this type of structure departs strongly from that expected for ionic bonding and ... whose roots go back to Max Planck's explanation in 1900 of the properties of ... types of interactions between elementary particles (the strong force, the weak force, ...